Special Risk:
Development of combustion gas vapours may be hazardous in the event of fire. Develop activities in the fire: hydrogen bromide.
Fire fighting equipment:
Try not to stay in the danger zone without self-breathing. How to avoid contact with skin, safe distance, and wear suitable protective clothing and helmets.
Other information:
Vapours out with water. Prevent the entry of fire-water or surface water.
Release action:
People associated with the prevention measures:
Menjahui substance contact. Not the vapours / aerosols. Stock Ensure fresh air in a closed space.
How to environmental protection:
Try not to let dirt enter the system.
The process of how to cleaning / absorption:
Make or take-absorbent material with a liquid (eg Chemizorb (C)). For disposal. Must clean the area already affected.
Handling and storage
Handling:
Working under the tent. Should not inhale substance. Avoid generation of vapours / aerosols.
Storage:
Tightly closed in well-ventilated place. Accesible only allowed to people. At 15 ° C
to 25 ° C.
Exposure controls / personal protection
Personal protection equipment:
Protective clothing should be selected specifically for the workplace, depending on the concentration and quantity of the hazardous substances handled.
Respiratory protection:
required when vapours / aerosols produced. Filter A (acc. to DIN 3181) for vapours of organic compounds.
Hand protection:
Full contact:
Glove material: viton
Layer thickness: 0.70 mm
Breakthrough time:> 480 Min.
In splash contact:
Glove material: butyl rubber
Layer thickness: 0.7 mm
Breakthrough time:> 30 Min.
Protective gloves should be used in accordance with the specifications of EC directive 89/686/EEC and the EN374 standard, for example, KCl 890 Vitoject (C) (full contact), 898 Butoject (C) (splash contact). Breakthrough times stated above were determined by laboratory tests in KCl Acc. to EN374 with samples of the type of gloves is recommended.
Industrial hygiene:
Immediately change contaminated clothing. Apply skin-protective barrier cream. Wash hands and face after working with substance. Under no circumstances eat or drink in the workplace. Working under the tent. Do not inhale substance.
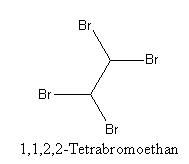
Physical and chemical properties
Forms: liquid
Color: red
Prestige: slightly sweet, spicy
pH value is not available
Melting point -1 ° C
Boiling point 239 ° C (decomposition)
Ignition temperature 335 ° C
Flash point not available
Lower explosion limit is not available
up is not available
Water vapor pressure (20 ° C) 0,03-0,13 PPA
Relative vapor density 11.92
Density (25 ° C) 2.96 g/cm3
Solubility in
water (20 ° C) 0.63 g / l
ethanol (20 ° C) soluble
Thermal decomposition 239-242 ° C
Bioconcentration factors 0,5-7,0
Stability and reactivity
Conditions to be avoided Heating. (Release from: hydrogen bromide). Materials to be avoided Violent reactions can be done with: / Risk of explosion with: alkali metals, alkaline earth metals, metals in the form of powder, sodium amide. Hazardous decomposition products in the event of fire.
More information
heat-sensitive, light-sensitive, contrary to various metals (iron, zinc, metal lighter), various plastics, rubber.
Toxicological information
3 acute toxicity
LC50 (inhalation, rat): 550 mg / m / 4 h.
LD50 (dermal, rat): 5250 mg / kg.
LD50 (oral, rat): 1200 mg / kg.
Literature to our data does not match the label specified by the European Commission. EC has dossiers which have not been published.
Subacute to chronic toxicity
Bacterial mutagenicity:
Ames test: positive.
Further toxicological information
After inhalation:
Irritations of the mucous membranes, cough, and dyspnea.
After skin contact:
Little irritations. Danger of skin absorption.
After eye contact:
Irritations.
After swallowing:
irritations of mucous membranes in the mouth, pharynx, esophagus and gastrointestinal tract.
Systemic effects:
Nausea, vomiting, headache.
After absorption of toxic quantities:
narcosis, coma, respiratory paralysis.
Effect / Damage:
liver, kidney.
Ecological information
Biological degradation:
Biodegradation: 29.0% / 14 d.
Biologically not easily degradable.
Behavior in environmental compartments:
Distribution: log p (o / w): 280.
Appreciable bioaccumulation potential that should be expected (log P (o / w)> 3).
Ecotoxic effects:
Biological effects:
Harmfull against the organism.
Description More Click Here











.jpg)